

The cookie is used to store the user consent for the cookies in the category "Analytics". This cookie is set by GDPR Cookie Consent plugin. These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. The double-bonded oxygen is electronegative, and attracts hydrogens. The structure of a carboxyl group can be seen below. Hydroxyl groups are simply an oxygen bonded to a hydrogen. The carboxyl group consists of a carbon, bonded to both an oxygen and a hydroxyl group. What is the structure of a carboxyl group? Another way to view it is as a carbonyl group (C=O) In chemistry, the carboxyl group is an organic, functional group consisting of a carbon atom that’s double-bonded to an oxygen atom and singly bonded to a hydroxyl group. All proteins are polymers of amino acids and all these acids except two have an amino group attached to the carbon atom next to the carboxyl group. The amino group (-NH2) is basic while the carboxyl group (- COOH ) is acidic in nature. An organic compound that binds to a carboxyl group is called a carboxylic acid.
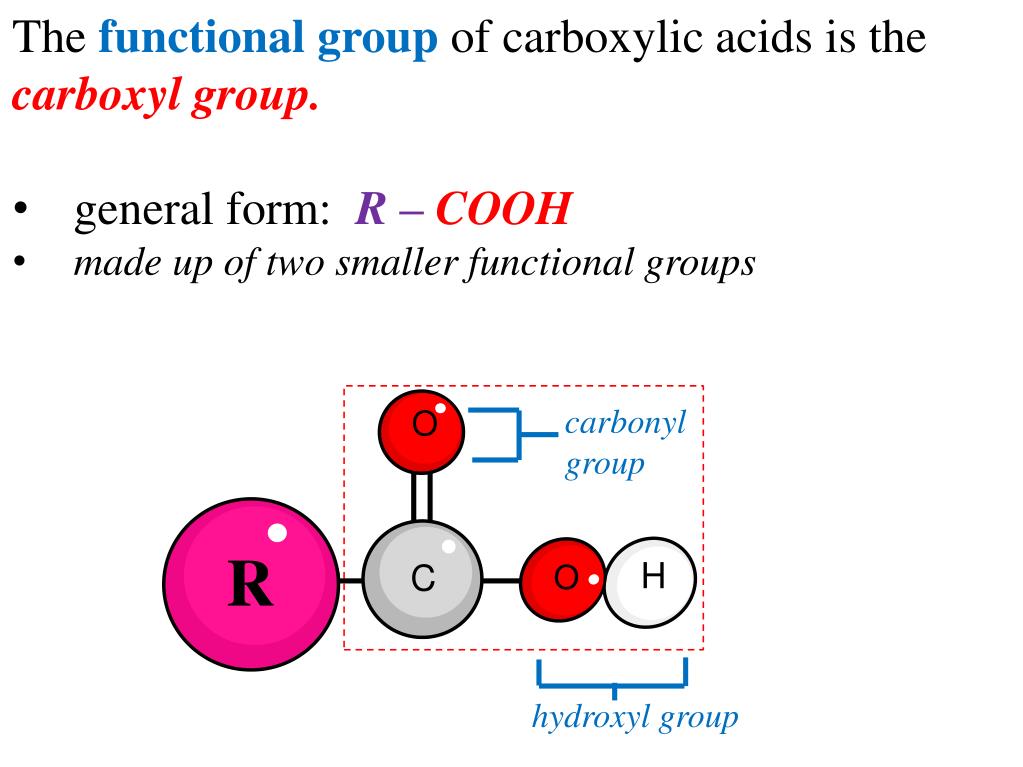
The carboxyl group is an important component of organic molecules such as amino acids, fatty acids, and acetic acids, all of which play essential roles in biosynthesis and cellular respiration. What is the function of the carboxyl group? An organic compound consisting of a carboxyl group is termed as a carboxylic acid. In a weak acid, few of the molecules are ionised.Ī Carboxyl Group is a functional organic compound that comprises a double-bonded carbon atom linked to an oxygen group and a hydroxyl group through a single bond. Their solutions do not contain many hydrogen ions compared to a solution of a strong acid at the same concentration. Why carboxylic acid is weak?Ĭarboxylic acids are weak acids because they only partially ionise in solution. Carboxylic acids are a class of molecules which are characterized by the presence of one carboxyl group. Is the carboxyl group a weak acid?Ĭarboxylic acids are referred to as “weak acids” because they partially dissociate in water.Ī carboxyl group (COOH) is a functional group consisting of a carbonyl group (C=O) with a hydroxyl group (O-H) attached to the same carbon atom. Since phosphate groups can release H+ ions into solution, they are considered acidic. Since amino groups can remove H+ from solution, they are considered basic. Since carboxyl groups can release H+ ions into a solution, they are considered acidic.
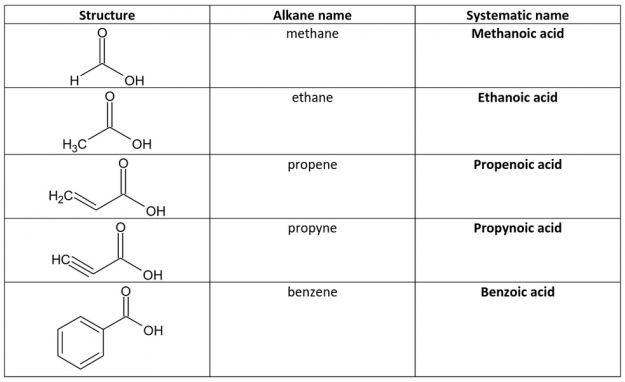
In aqueous solution, the O−H bond of the hydroxyl group can break, yielding a negative carboxylate ion and the hydrogen ion. 5 Is a carboxyl group a functional group?Ĭarboxylic acids are all weak acids.4 What is the function of the carboxyl group?.1 Is the carboxyl functional group acidic?.


 0 kommentar(er)
0 kommentar(er)
